Knee implants
all procedures accredited
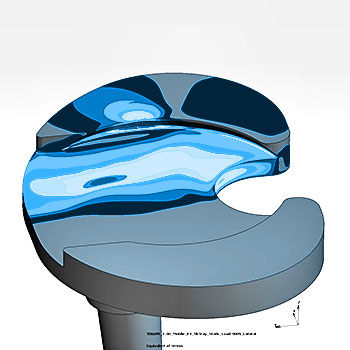
Tibial Component Finite Element Analysis - ASTM F3334
Normative References
ASTM F3334: Standard Practice for Finite Element Analysis (FEA) of Metallic Orthopaedic Total Knee Tibial Components
Safety against fatigue fractures of tibial components of total knee replacements is typically determined according to ASTM F1800 or ISO 14879. ASTM F3334 is an excellent tool for worst-case assessment within a series of different implant sizes of the same implant design to reduce the physical test burden. Specific model verification is based on ASME V&V 40 with special focus on the geometry and in-vivo loading of the tibial component.